Clinical Evaluations
NexxZr T (6 mo)
Description
Purpose:
The purpose of this clinical study was to determine the clinical performance of NexxZr T (Sagemax) full-contour zirconia restorations at placement and at six-month recall. The restorations were fabricated by Apex Dental Milling and Nellmar Laboratory.
Placement
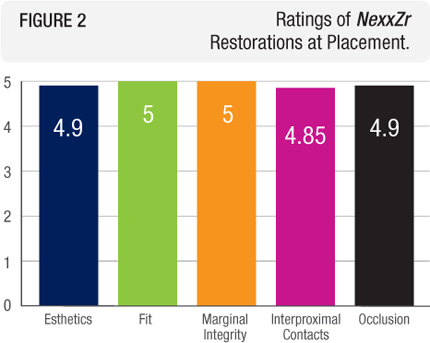
One hundred sixty full-contour NexxZr T crowns were evaluated at placement for:
• Esthetics
• Fit
• Marginal integrity
• Contacts
• Occlusion
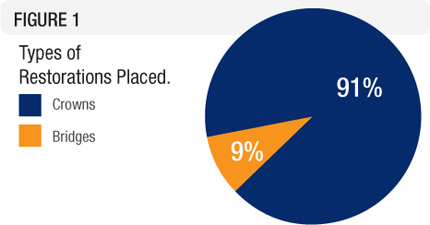
The restorations included 146 posterior single crowns; two, three-unit posterior bridges; and one, eight-unit anterior bridge (Figure 1). The restorations were cemented with adhesive resin cement or self-adhesive resin cement. Restorations were evaluated on a 1-5 rating scale:
1 = poor, 2 = fair, 3 = good, 4 = very good, 5 = excellent.
Esthetics, fit, marginal accuracy, interproximal contacts, and occlusion of all of the restorations were rated excellent at placement (Figure 2). Seven restorations required minor occlusal adjustment, whereas eight restorations had light interproximal contacts.
Results at Six Months
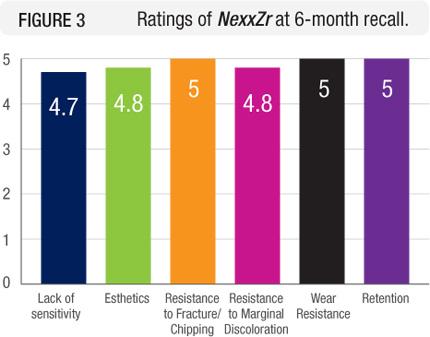
In December, 2012, 25 NexxZr T restorations were recalled and evaluated. The recalled NexxZr T restorations were evaluated in the following categories:
• Lack of sensitivity
• Esthetics
• Resistance to fracture/chipping
• Resistance to marginal discoloration
• Wear resistance of zirconia and opposing dentition
• Retention
Restorations were evaluated on a 1-5 rating scale: 1 = poor, 2 = fair, 3 = good, 4 = very good, 5 = excellent.
Esthetics, resistance to fracture or chipping, resistance to marginal discoloration, and wear resistance of the restoration and opposing dentition rated excellent (Figure 3). A few patients experienced minor to moderate postoperative sensitivity. None of the restorations debonded.
Conclusions
All of the 160 NexxZr T restorations rated excellent at placement. Minor occlusal adjustment was required for 4% of the restorations. At six-month recall, all of the 25 restorations were rated excellent. None of the restorations had fractured or chipped. A few patients reported minor to moderate sensitivity. None of the restorations debonded. NexxZr T received a 98% clinical rating at the six-month recall.

